Temonat (temozolomide) for Injection
Temonat is a brand-name prescription drug that’s FDA-approved to treat brain tumors such as glioblastoma and anaplastic astrocytoma. Temonat (temozolomide) for Injection administered via intravenous infusion Initial U.S. Approval: 1999
Home | (temozolomide) for Injection
Submit a request to access drug used to treat, diagnose or prevent serious or life-threatening conditions.We’ll get back to you within 1 business days!
For Overseas Patient/ Export inquires.
Call / WhatsApp Number
+91 9643240915 (Mr. Yashpal Singh)
+91 9289711087 (Ms. Sana Sadyar)
For any urgent inquiries or assistance needed, please reach out to our Support Team. Rest assured, we will respond within 24 hours, from Monday to Saturday, during the hours of 9:00 A.M to 18:00 PM.
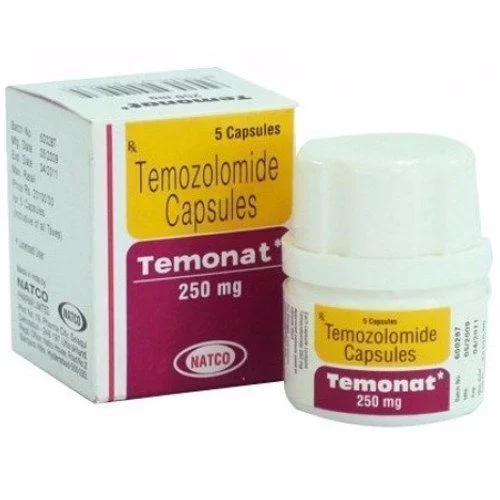
TEMONAT (temozolomide) for Injection
Temozolomide is approved to treat the following types of brain tumors in adults:Anaplastic astrocytoma, Glioblastoma multiforme (GBM).
Temonat 100mg 5 Capsule belongs to a class of medicines called ‘anti-cancer/ antitumor agent’, used to treat specific forms of brain tumours such as glioblastoma multiforme (a type of tumour that grows and spreads rapidly) in adults and malignant glioma, including glioblastoma multiforme or anaplastic astrocytoma (a rare malignant brain tumour) in patients older than 3years. Temonat 100mg 5 Capsule contains Temozolomide which works by inhibiting the production of DNA (deoxyribonucleic acid-genetic material of the cell), thereby affecting cell growth and division. Thus, Temonat 100mg 5 Capsule prevents tumours from growing.
Temozolomide, A drug used to treat adults with certain types of brain tumors. It is also being studied in the treatment of other types of cancer. Temozolomide damages the cell’s DNA and may kill cancer cells. It is a type of alkylating agent. Also called Temodar.
In the United States temozolomide is indicated for the treatment of adults with newly diagnosed glioblastoma multiforme concomitantly with radiotherapy and subsequently as monotherapy treatment; or adults with refractory anaplastic astrocytoma who have experienced disease progression on a drug regimen containing nitrosourea and procarbazine.
In the European Union temozolomide is indicated for adults with newly diagnosed glioblastoma multiforme concomitantly with radiotherapy and subsequently as monotherapy treatment; or children from the age of three years, adolescents and adults with malignant glioma, such as glioblastoma multiforme or anaplastic astrocytoma, showing recurrence or progression after standard therapy.
Drug (Brand / Generic): Temonat / temozolomide for Injection
Current Indications: brain tumors
Marketed by:: NATCO PHARMA LIMITED
Approval Date: 2015
Available as (Form & Strength): 5-mg, 20-mg, 100-mg, 140-mg, 180-mg, and 250-mg capsules
For more highlights of prescribing information regarding Indications and usage, dosage and administration, dosage forms and strengths, patient counseling information and medication guide. Click Here
Alleviare Life Sciences
About Us
Alleviare Life Sciences Pvt. Ltd. (India) serves as a facilitator for medical and pharmaceutical products, commencing its operations in 2005. Headquartered in Delhi, India, Alleviare operates nationwide, providing a range of comprehensive services to patients, doctors, hospitals, and government healthcare facilities.
Our specialization lies in facilitating access to medicines, leveraging our expertise in sourcing and providing documentation assistance for programs such as Named Patient Program, Managed Access Program, and Early Access Program. We take pride in ensuring smooth and efficient access to medications for those who require them.

Alleviare India is certified pharmaceutical facilitator / supplier /exporter based in india. They are also who GDP certified. One of the pharmaceutical products they offer is “ Temonat (temozolomide) for Injection ”. Confirmation of the order for Temonat (temozolomide) for Injection will be subject to the submission of a valid doctor’s prescription and, if applicable, an export permit.
Get Access to Treatment – temozolomide
We have delivered Temonat (temozolomide) for Injection to following countries –
Argentina, Australia, Austria, Azerbaijan, Bahrain, Brazil, Bulgaria, Cambodia, Canada, Chile, Colombia, Costa Rica, Croatia, Cyprus, Denmark, Dominican Republic, Estonia, Finland, France, Georgia, Germany, Ghana, Greece, Guatemala, India, Iraq, Ireland, Israel, Italy, Jamaica, Japan, Jordan, Kenya, Kuwait, Latvia, Lebanon, Libya, Lithuania, Malawi, Mexico, Montenegro, Nepal, Netherlands, New Zealand, Nigeria, Norway, Oman, Pakistan, Paraguay, Peru, Poland, Qatar, Romania, Saudi Arabia, Serbia, Singapore, Slovenia, Spain, Sri Lanka, Sweden, Switzerland, United Arab Emirates, United Kingdom, United States, Venezuela, Zimbabwe.
To place an order, you can order online, call us at M/W: +91 9289711087 / 9289711088. You can also make an inquiry by emailing us at contact@alleviareindia.com
Wholesale Generic Drug Supplier
Alleviare provides generic drugs at wholesale prices to the entire India. We are a pharmaceutical wholesaler that works with a large variety of pharmaceutical manufacturers and buy in bulk. As a result, this allows us to offer you generic drugs at a wholesale price. In addition, we supply long-term care pharmacies, retail pharmacies, specialty pharmacies, and compounding pharmacies nationwide.
Alleviare India specializes in sourcing and supplying Temonat, a cancer treatment medicine, from around the world. They offer worldwide access to the best available treatment options.
How much does Temonat cost in the India?
Kindly reach out to us for Temonat (temozolomide) for Injection price and availability confirmation. The price of the medicines is the cost set by the manufacturer.
To place an order, you can order online, call us at M/W: +91 9289711087 / 9289711088. You can also make an inquiry by emailing us at contact@alleviareindia.com
Is temozolomide available in India?
Yes temozolomide is available in India. Temonat letermovir is a prescription medication, which means it legally requires a medical prescription to be dispensed. ALS can facilitate the supply of Temonat (prescription medicines) to locations worldwide, including India, while ensuring compliance with legal requirements. Please contact or mail @ – info@alleviareindia.com +91 9289711087 / 9289711088 for Temonat availability and prices in India. We guarantee the authenticity and quality of our products and offer worldwide delivery according to the buyer’s specifications.
How overseas patient can get access?
ALS serves as a facilitator, supplier & exporter in India assisting patients, doctors, and hospitals in importing the prescription medicine brand Temonat (temozolomide) for Injection . The import process requires a valid prescription and an Import License in the Patient’s Name.
For patients from following foreign countries seeking access to Temonat (temozolomide) for Injection , they can inquire and find further information by sending their inquiries to ALS.
To place an order, you can order online, call us at M/W: +91 9289711087 / 9289711088. You can also make an inquiry by emailing us at contact@alleviareindia.com

